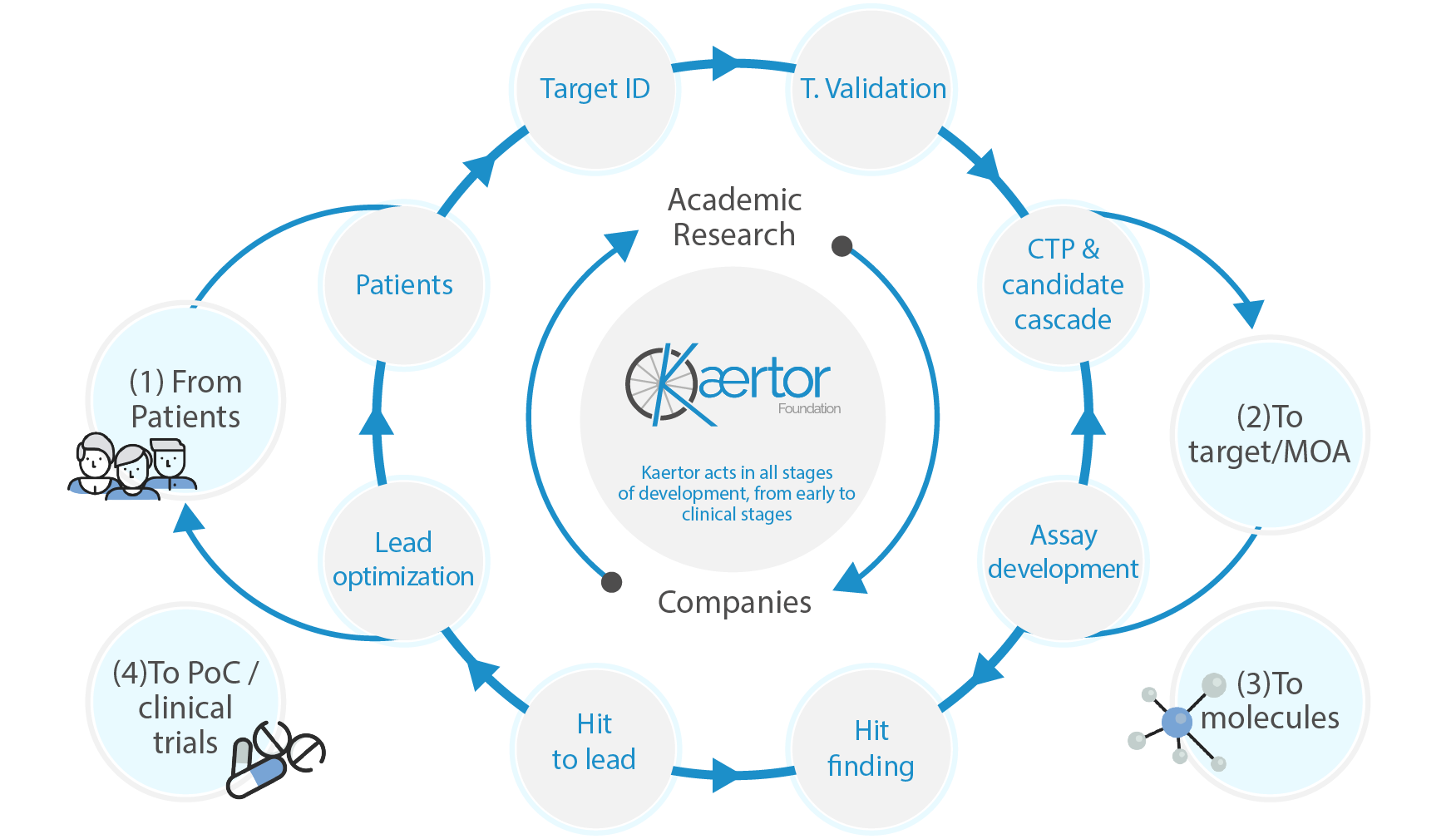
Our mission is to generate, manage, execute and promote drug discovery programs and the social and productive economy of this sector in Galicia. The Foundation’s activities are aimed at the reciprocal transfer of research results in the context of open innovation in drug discovery.
This website uses cookies so that we can offer you the best possible user experience. The cookie information is stored in your browser and performs functions such as recognizing you when you return to our website or helping our team to understand which sections of the website you find most interesting and useful.